- AIMS Newsletter
- Posts
- When doctors say 'never' — but MS patients say 'watch me'
When doctors say 'never' — but MS patients say 'watch me'

Another week, another step forward in our mission to help MS patients access HSCT.
This week:
The patient who went from paralyzed to walking despite doctors' predictions
What fenebrutinib actually means for MS patients
How close we are to our next funding milestone

💪 "They Said I'd Never Walk Again"

What would you do if doctors told you your legs would never work again?
One of our grant recipients heard exactly that in February 2022.
Her journey: Misdiagnosed for years (fibromyalgia, Lyme disease). Finally got her MS diagnosis in December 2020. Tried Tecfidera, Aubagio, then Ocrelizumab.
Then came October 2021. A massive relapse left her completely paralyzed from the waist down. Stuck in her bedroom for four months. Transferred to rehab in February 2022.
The doctors said: "You won't walk again."
But she spent six months in that rehab hospital fighting. Refusing to believe them. Doing the impossible work.
And she proved them wrong. Today, she can walk short distances with an aid.
Because when conventional treatments stop working, when you're told you've reached your limit, HSCT says: "Let's try something different."
If you're stuck in your own version of "you'll never..." — you're not alone. And you don't have to accept that as your final answer.

The New Drug Everyone's Talking About (And What It Actually Means)
You've probably seen headlines about Roche's new MS drug fenebrutinib making waves.
Here's what you need to know:
Two weeks ago, Roche announced their oral BTK inhibitor succeeded in Phase III trials for both relapsing MS AND primary progressive MS — the first to succeed where competitors failed.
What's different? Most MS drugs target B-cells in your blood. BTK inhibitors cross the blood-brain barrier and target inflammation inside your central nervous system, including brain immune cells (microglia) that drive chronic progression.
The results:
Significantly reduced relapse rates vs. Aubagio
Non-inferior to Ocrevus for primary progressive MS
Near-complete suppression of disease activity over 96 weeks
So why aren't we jumping for joy?
Because we've been here before. Every new drug gets announced with fanfare and "promising results."
The reality:
Not approved yet (regulatory submission expected first half of 2026)
Had liver safety concerns (FDA clinical hold in December 2023)
Even with "near-complete suppression," some disease activity still occurred
Will fenebrutinib help some people? Probably. Should you be excited? Absolutely.

We are getting there…….
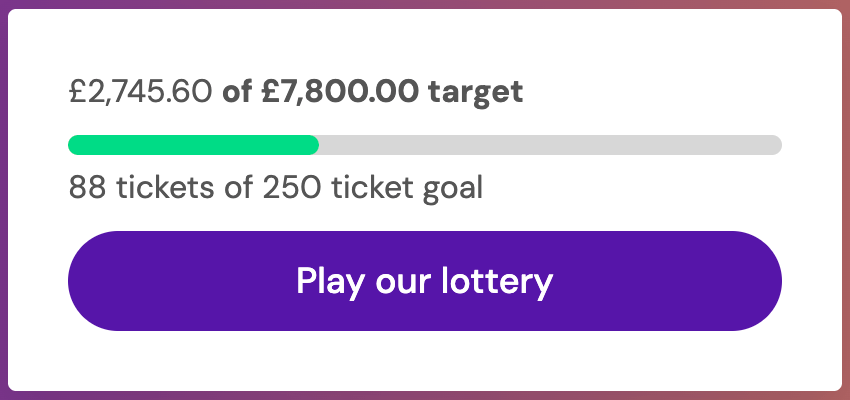
We're at £2,745.60 of our £7,800 target.
162 tickets sold of our 250-ticket goal.
That gap isn't just a number. It's the difference between telling someone "we can help you" and "not yet."
Why share this?
Because raising awareness isn't just about ticket sales — it's about reaching the MS patient who doesn't know AIMS exists yet.
The person scrolling right now, researching options, feeling isolated. Your colleague's sister, your neighbor's friend who just got diagnosed and is trying to figure out what's next.
When you share The MS Big Draw, you're doing two things:
Helping us fund more patients
Introducing someone to HSCT who might not have heard of it
The details:
£1 per ticket
£25,000 top prize
1 in 50 chance to win (45x better odds than national lottery)
Drawn every Saturday at 8pm
60% goes directly to AIMS
100% supports HSCT access
If you can't buy a ticket, share it. If you can buy one, share it anyway.
Every share reaches someone we haven't reached yet. And that person might be the one who needs us most.
You're not alone in this. We'll be in your inbox next week — one step, one story, one answer at a time.
SIGNING OFF... with strength 💪
— AIMS Team